Debate with a Fizz Killer...
A few days ago, I got into a heated debate with some co-workers (not as heated as the one about “white chocolate”) one day because one of them had purchased a 20 ounce bottle of Coca-Cola and after drinking about 20% of the contents he proceeded to squeeze the bottle then recap it.

“Homer, what is your rationale for doing that?” I asked.
Homer said, “Duh….If you have a partially full bottle of soda, and you squeeze the sides together, it creates less air in the bottle and the soda won’t go flat as fast.
At first I thought I could understand how this could work. I thought that it would have something to do with partial pressure. But after reasoning this concept through, I figured that there was more to it than that. So…What IS the best way to keep Homer’s soda from going flat?
After putting on my Physics brain…Here is my take on this:
Squeezing the bottle, as Homer did, and then capping it will produce a less than 1 atmosphere pressure condition inside the bottle because the flexible container will try to return to its original shape. After digging around in some old Physics books I found our old friend...
Le Châtelier's Principle of Chemical Equilibrium, which goes something like this:
If an outside influence upsets an equilibrium, the system undergoes a change in a direction that counteracts the disturbing influence and, if possible, returns the system to equilibrium.
The three methods to stress a system that's in chemical equilibrium -
1. a change in concentration(s)
2. a change in temperature
3. a change in pressure
In this situation, involving the squeezing of the soda bottle, we are concerning primarily with number 3. The concentration isn't changing substantially (yep...I'm calling Homer's backwash negligible) and to keep it simple, neither is the temperature, even though the soda is slowly warming up to room temperature.
The inverse relationship between volume and gas pressure is the piece of the puzzle we need. We need to first consider how the volume change will affect pressure and then consider how the change in pressure affects the chemical equilibrium. This leads us to Boyle's Law which will give us a relationship between pressure and volume. Increase volume and pressure is decreased. Decrease volume and pressure is increased.
Boyle's Law
Pressure is inversely propertional to the volume:

And this gives us Boyle's Law:
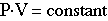
The reduced pressure inside the squeezed bottle will encourage the carbonation to be released from solution. Looking at the carbonic acid (yep...you are drinking carbonic acid...read your bottle...mmmm...mmmm...tasty!) equilbrium equation, the reaction shifts to the side that produces more gas molecules:
This will make the soda go flat sooner. So Homer, you are actually killing the fizz. You are a fizz killer!
The bottom line is…You will be more successful in keeping the soda from losing its fizz, by capping it tightly and keeping it cold at an elevated pressure. There is also a special cap out on the market that lets you re-pressurize the bottle. You just screw it in place and then operate a little pump that is part of and affixed to the center of the cap. I know this sounds pretty trivial, but it bothered me enough to waste about 20 minutes of brain power.
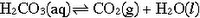








0 Comments:
Post a Comment
<< Home